The China Products Regulatory Consultant and Services Provider
- WeChat:China_PSRA
- Hotline 24H: 86 1391 086 3852
- oci.services@oci.net.cn

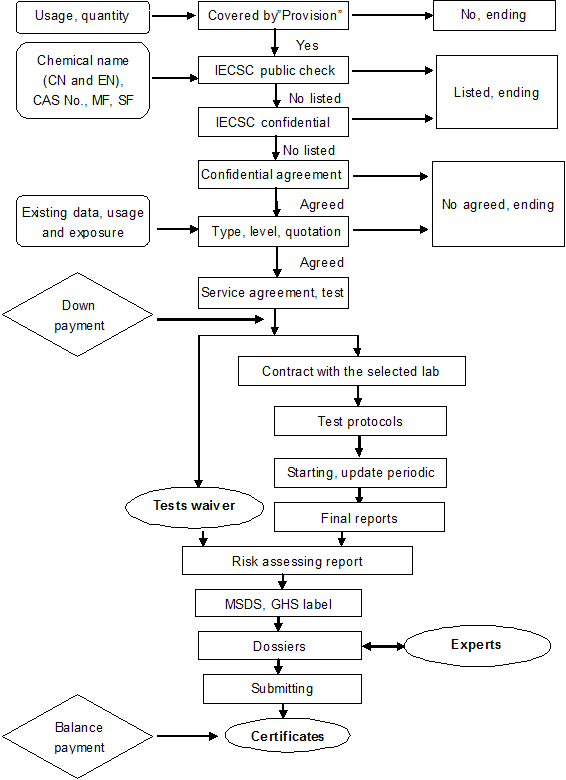
In order to enhance the environmental management and control the environmental risk on new chemical substances, prevent environmental pollution, and protect human health and safeguard the ecological environment, MEP issue the “Provisions on the Environmental Administration of New Chemical substances”. According to the Provision, “new chemical substance” means any chemical substance which has not yet been listed in the Inventory of Existing Chemical Substances Manufactured or Imported in China (IECSC). Any manufacturer or importer of new chemical substances shall, prior to the manufacture or import of new chemical substances, make notifications and applications for the registration certificates for the environmental management on new chemical substances (hereinafter cited as ‘the registration certificates’) in compliance with this Provisions.
General Notification
Simple Notification
Scientific and Research Record Notification
(Ministry of Environmental Protection of the People’s Republic of China Statute No.7)
2 Solid Waste and Chemicals Management Technology Centre of MEE, SCC-MEESolid Waste and Chemicals Management Technology Centre of MEP was established in June 2013. It was formed by combining the former solid waste management center of MEP and the former Chemical Registration Center of MEP. Its name was changed to Solid Waste and Chemicals Management Technology Centre of MEE in May 2019. It is a bureau level institution directly under MEE. It is a technique supporting sub-institute for management of solid waste, chemicals, contaminated sites and heavy metal environment of MEE.
3 OCI NCSN Department and TeamOCI is one of the first companies to focus on new chemical substances notification (NCSN), and has accumulated rich experience.
In the actual operation of many years, the company has successfully established a sophisticated core technology team, covering all aspects of the business, able to give feedback to the customer's information in time, meeting the various needs of the customers from different angles, and attracting more and more attention in the rapid development of China and the petrochemical industry. OCI provides you with excellent new chemical substance notification service.
4 Advantages of OCIThe optimum experiment plan, the shortest processing time and the best results are from:
(1) Our professional team and long-term stable partner relationships with well-known laboratory at home and abroad
(2) The support of hygeian toxicology and ecotoxicology experts at home and abroad
(3) Close relationship with relevant authorities
(4) Abundant data resources
(5) The perfect commercial secrecy system
| Items | Description |
IECSC check |
Public or confidential |
Initial assessment |
Assess the notification type via the usage, quantity and Sponsor |
Notification plan |
Base on the existing data, notification type and exposure information |
Periodic report |
Update by month or quarter |
Translating reports |
Review the acceptability |
Translating the summary |
|
Arranging the tests |
Different price level labs for choice |
Contract with the selected lab |
|
Update by month or quarter |
|
Review the test results |
|
Send the test results to the Sponsor |
|
Test waiver |
Base on the existing data, usage and exposure information |
Risk assessing report |
Propose the control measures via the physicochemical, toxicity and eco-toxicity data, usage and exposure information, |
Notification dossiers |
Preparing the test data |
Compiling the environmental risk assessing report |
|
Compiling or translating MSDS, GHS labels |
|
Submitting the dossiers |
|
MSDS, GHS labels |
Based on the existing data and original version, in compliance with Chinese regulations |
| OECD No. | Chinese Name | English Name |
| Section 1 Physis-Chemical Properties | ||
| 101 | 紫外可见吸收光谱 | UV-VIS Absorption Spectra |
| 分光光度法 | Spectrophotometric Spectra | |
| 102 | 熔点/熔点范围 | Melting Point/Melting Range |
| 103 | 沸点 | Boiling Point |
| 104 | 蒸汽压 | Vapour Pressure |
| 105 | 水溶解度 | Water Solubility |
| 106 | 吸附/解吸 | Adsorption/Desorption |
| 107 | 分配系数(正辛醇/水)-摇瓶法 | Partition Coefficient (n-octanol/water)-Shake Flask Method |
| 108 | 在水中形成配位化合物的能力-极谱法 | Complex Formation Ability in Water-Polarographic Method |
| 109 | 液体和固体的密度 | Density of Liquids and Solids |
| 110 | 颗粒物粒度分布/纤维长度和直径分布 | Particle Size Distribution/Fibre Length and Diameter Distributions |
| 111 | 与pH有关的水解作用 | Hydrolysis as a Function of pH |
| 112 | 在水中的离解常数 | Dissociation Constants in Water |
| 113 | 热稳定性和空气中稳定性的筛选试验 | Screening Test for Thermal Stability and Stability in Air |
| 114 | 液体的粘度 | Viscosity of Liquids |
| 115 | 水溶液的表面张力 | Surface Tension of Aqueous Solution |
| 116 | 固态和液态物质的脂溶性-烧瓶法 | Fat Solubility of Solid and Liquid Substances-Flask Method |
| 117 | 分配系数(正辛醇/水)-高效液相色谱法 | Partition(n-octanol/water),High Performance Liquid Chromatography(HPLC)Method |
| 118 | 凝胶渗透色谱法(GPC)测定聚合物的数均分子量及分子量分布 | Determination of the Number Average Molecular Weight and the Molecular Weight Distribution of Polymers Using Gel Permeation Chromatography |
| 119 | 凝胶渗透色谱法(GPC)测定聚合物低分子量部分的含量 | Determination of the Low Molecular Weight Content of a Polymer Using Gel Permeation Chromatography |
| 120 | 聚合物在水中的溶解/萃取行为 | Solution/Extraction Behaviour of Polymers in Water |
| Section 2 Effects on Biotic Systems | ||
| 201 | 藻类生长抑制试验 | Alga Growth Inhibition Test |
| 202 | 溞类24h EC50急性活动抑制试验 | Daphnia sp.24h EC50 Acute Immobilization Test |
| 203 | 鱼类急性毒性试验 | Fish Acute toxicity Test |
| 204 | 鱼类14天延长毒性试验 | Fish, Prolonged Toxicity Test:14-day Study |
| 205 | 鸟类日粮毒性试验 | Avian Dietary Toxicity Test |
| 206 | 鸟类繁殖试验 | Avian Reproduction Test |
| 207 | 蚯蚓急性毒性试验 | Earthworm, Acute Toxicity Test |
| 208 | 陆生植物生长试验 | Terrestrial Plants, Growth Test |
| 209 | 活性污泥呼吸抑制试验 | Activated Sludge, Respiration Inhibition Test |
| 210 | 鱼类早期生活阶段毒性试验 | Fish, Early-life Stage Toxicity Test |
| 211 | 大型溞繁殖试验 | Daphnia magna Reproduction Test |
| 212 | 鱼类胚胎-卵黄囊吸收阶段的短期毒性试验 | Fish, Short-term Toxicity Test on Embryo and sac-fry Stages |
| 213 | 蜜蜂急性经口毒性试验 | Honeybees, Acute Oral Toxicity Test |
| 214 | 蜜蜂急性接触毒性试验 | Honeybees, Acute Contact Toxicity Test |
| 215 | 鱼类幼体生长试验 | Fish, Juvenile Growth Test |
| 216 | 土壤微生物:氮转化测试 | Soil Microorganisms: Nitrogen Transformation Test |
| 217 | 土壤微生物:碳转化测试 | Soil Microorganisms: Carbon Transformation Test |
| 299 | 种子发芽和根伸长毒性试验 | Seed Germination/Root Elongation Toxicity Test |
| Section 3 Degradation and Accumulation | |||
| 301 | 快速生物降解性 | Ready Biodegradability | |
| 301 | 301A | DOC消减试验 | DOC Die-Away Test |
| 301B | CO2产生试验 | CO2 Evolution Test | |
| 301C | 改进的MITI试验(I) | Modified MITI Test I | |
| 301D | 密闭瓶试验 | Closed Bottle Test | |
| 301E | 改进的OECD筛选试验 | Modified OECD Screening Test | |
| 301F | 呼吸计量法试验 | Manomettric Respiremetry Test | |
| 302 | 302A | 改进的半连续活性污泥(SCAS)试验 | Inherent Biodegradability: Modified SCAS Test |
| 302B | 赞恩-惠伦斯试验 | Zahn-Wellens Test | |
| 302C | 改进的MITI试验(II) | Inherent Biodegradability: Modified MITI Test II | |
| 303A | 模拟试验—好氧污水处理:偶联单元试验 | Simulation Test-Aerobic Sewage Treatment: Coupled Units Tests | |
| 304A | 土壤固有生物降解能力 | Inherent Biodegradability in Soil | |
| 305 | 流水式鱼类试验 | Bioconcentration: Flow-Through Fish Test | |
| 305 | 305A | 连续静态鱼类试验 | Bioconcentration: Sequential Static Fish Test |
| 305B | 半静态鱼类试验 | Bioconcentration: Semi-Static Fish Test | |
| 305C | 鱼类生物富集试验 | Bioconcentration: Test for the Degree of Bioconcentration in Fish | |
| 305D | 静态鱼类试验 | Bioconcentration: Static Fish Test | |
| 399 | 吸收和蓄积试验 | Update and Accumulation Test | |
| Section 4 Health Effects | |||||
| 401 | 急性经口毒性试验 | Acute Oral Toxicity Test | |||
| 402 | 急性经皮毒性试验 | Acute Dermal Toxicity Test | |||
| 403 | 急性吸入毒性试验 | Acute Inhalation Toxicity Test | |||
| 404 | 急性皮肤刺激性/腐蚀性试验 | Acute Dermal Irritation/Corrosion Test | |||
| 405 | 急性眼刺激性/腐蚀性试验 | Acute Eye Irritation/Corrosion Test | |||
| 406 | 皮肤致敏试验 | Skin Sensitization Test | |||
| 407 | 啮齿类动物28天反复经口毒性试验 | Repeated Dose 28-day Oral Toxicity Study in Rodents | |||
| 408 | 啮齿类动物亚慢性(90天)经口毒性试验 | Repeated Dose 90-day Oral Toxicity Study in Rodents | |||
| 409 | 非啮齿类动物亚慢性(90天)经口毒性试验 | Repeated Dose 90-day Oral Toxicity Study in Nonrodents | |||
| 410 | 反复经皮毒性:21/28天试验 | Repeated Dose Dermal Toxicity:21/28-day Study | |||
| 411 | 亚慢性经皮毒性:90天试验 | Subchronic Dermal Toxicity:90-day Study | |||
| 412 | 反复吸入毒性:28天或14天试验 | Repeated Dose Inhalation Toxicity:28/14-day Study | |||
| 413 | 亚慢性吸入毒性:90天试验 | Subchronic Inhalation Toxicity:90-day Study | |||
| 414 | 致畸试验 | Teratogenicity | |||
| 415 | 一代繁殖毒性试验 | One-Generation Reproduction Toxicity Study | |||
| 416 | 两代繁殖毒性试验 | Two-Generation Reproduction Toxicity Study | |||
| 417 | 毒代动力学试验 | Toxicokinetics Study | |||
| 418 | 有机磷化合物急性染毒的迟发性神经毒性试验 | Delayed Neurotoxicity of Organophosphorus Substances Following Acute Exposure | |||
| 419 | 有机磷化合物亚慢性(28天)染毒的迟发性神经毒性试验 | Delayed Neurotoxicity of Organophosphorus Substances:28-Day Repeated Dose Study | |||
| 420 | 急性经口毒性:固定剂量法 | Acute Oral Toxicity-Fixed Dose Method | |||
| 421 | 生殖和发育毒性筛选试验 | Reproduction/Developmental Toxicity Screening Test | |||
| 422 | 结合反复染毒毒性研究的生殖发育毒性筛选试验 | Combined Repeated Dose Toxicity Study with the Reproduction/Developmental Toxicity Screening Test | |||
| 423 | 急性经口毒性:急性毒性的阶层法 | Acute Oral Toxicity-Acute Toxic Class Method | |||
| 424 | 啮齿类动物的神经毒性试验 | Neurotoxicity Study in Rodents | |||
| 425 | 急性经口毒性:上下增减剂量法 | Acute Oral Toxicity-Up and Down Procedure | |||
| 451 | 致癌试验 | Carcinogenicity Studies | |||
| 452 | 慢性毒性试验 | Chronic Toxicity Studies | |||
| 453 | 慢性毒性与致癌性联合试验 | Combined Chronic Toxicity/Carcinogenicity Studies | |||
| 471/472 | 细菌回复突变试验 | Bacterial Reverse Mutation Test | |||
| 473 | 体外哺乳动物细胞染色体畸变试验 | In vitro Mammalian Chromosome Aberration Test | |||
| 474 | 哺乳动物红细胞微核试验 | Mammalian Erythocyte Micronucleus Test | |||
| 475 | 哺乳动物骨髓染色体畸变试验 | Mammalian Bone Marrow Chromosome Aberration Test | |||
| 476 | 体外哺乳动物细胞基因突变试验 | In vitro Mammalian Cell Gene Mutation Test | |||
| 477 | 黑腹果蝇伴性隐性致死试验 | Sex-linked Recessive Lethal Test in Drosophila Melanogaster, SLRL | |||
| 478 | 啮齿类动物显性致死试验 | Rodent Dominant Lethal Test | |||
| 479 | 哺乳类动物细胞姐妹染色单体互换体外试验 | In vitro Sister Chromatid Exchange Assay in Mammalian Cells |
|||
| 480 | 酿酒酵母基因突变试验 | Saccharomyces cerevisiae, Gene Mutation Assay | |||
| 481 | 酿酒酵母有丝分裂重组试验 | Saccharomyces cerevisiae Mitotic Recombination Assay | |||
| 482 | 哺乳类动物细胞DNA损害与修复/程序外DNA合成体外试验 | DNA Damage and Repair/Unscheduled DNA Synthesis in Mammalian Cells in vitro | |||
| 483 | 哺乳动物精原细胞染色体畸变试验 | Mammalian Spermatogonial Chromosome Aberration Test | |||
| 484 | 小鼠斑点试验 | Mouse Spot Test | |||
| 485 | 小鼠可遗传易位试验 | Mouse Heritable Translocation Assay | |||
| 486 | 体外哺乳动物肝细胞程序外DNA合成(UDS)试验 | Unscheduled DNA Synthesis (UDS)Test with Mammalian Liver Cells in vitro | |||
| 490 | 空斑形成细胞试验 | Plaque Forming Cell Assay | |||
| 491 | 迟发性超敏反应试验 | Delayed Type Hypersensitivity Test | |||
| 492 | 自然杀伤细胞活性试验 | Natural Killer Cell Activity Assay | |||